Draw A Water Molecule And Label The Partial Charges On The Molecule

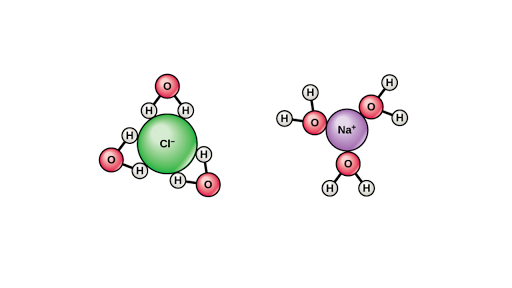
For two formula units of nacl drag the sodium ions and chloride ions to where they would most likely appear based on the grouping of the water molecules in the area provided.
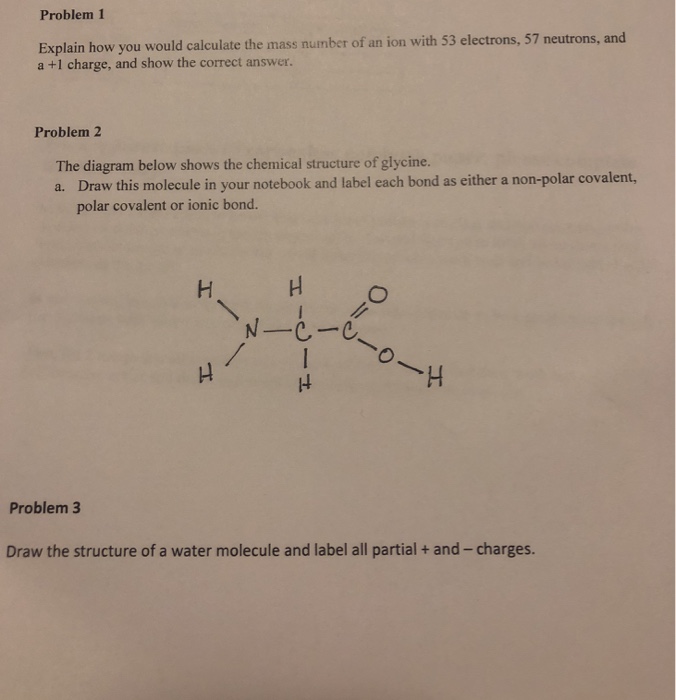
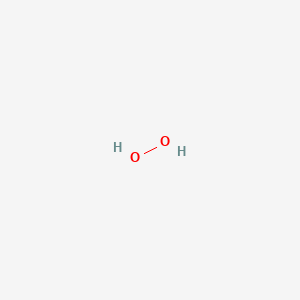
Draw a water molecule and label the partial charges on the molecule. This is actually a really hard thing to do. Nonpolar covalent bond b. Draw the structure of a single water molecule showing any charges that may exist and label of the types of bonds that are present what is a hydrogen bond a type of attractive interaction between in electronegative atom and a hydrogen atom.
Draw a picture below showing four water molecules interacting via hydrogen bonds. H h o somewhat like so accept a bit larger. Polar covalent bond 15.
Which type of covalent bond makes a molecule more hydrophilic and water soluble. Be sure to label partial charges in each molecule and use a dotted line to represent a hydrogen bond. Label the partial charges within each water molecule.
The slightly negative oxygen atom of one molecule is attracted to the slightly positive hydrogen atom of the other water molecule and this. Water is a polar molecule meaning it carries partial charges δ or δ on opposite sides of the molecule. Water is a tiny bent molecule with the molecular formula h 2 o consisting of two light hydrogen atoms attached to each 16 fold heavier oxygen atom.
Draw a water molecule involved in hydrogen bonds with three other water molecules. Using structural formula diagrams. Water is a molecular compound.
It is very tricky for the human mind to even imagine a water molecule with any degree of accuracy let alone successfully draw it. It is a polar molecule in which the oxygen end of the molecule has a partial negative charge and the hydrogen end of the molecule has a partial positive charge. We know that a water molecules comprises three atoms 2 x h 1 x o so.
This property allows one water molecule to form hydrogen bonds with three other water molecules. This clip is a chalkboard lecture that explains why water is a polar molecule and how its polarity affects its interaction with other molecules. To draw the lewis structure for water one must draw two capital hs about 2 inches appart.
Asked feb 3 2020. Each molecule is electrically neutral but polar with the center of positive and negative charges located in different places.