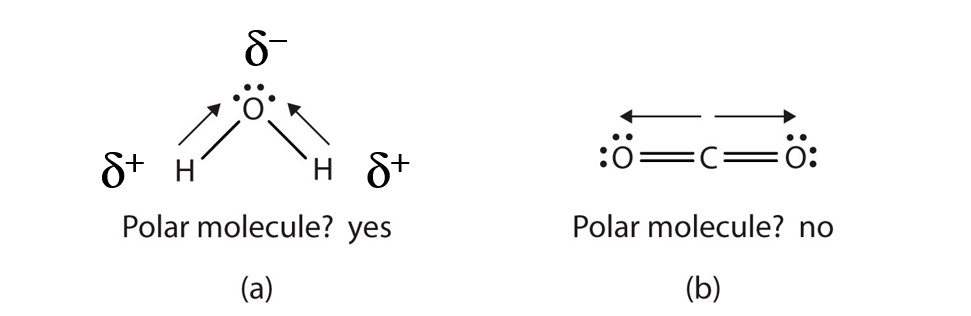Draw A Water Molecule And Include Partial Negative And Positive Charges

Water is a tiny bent molecule with the molecular formula h 2 o consisting of two light hydrogen atoms attached to each 16 fold heavier oxygen atom.
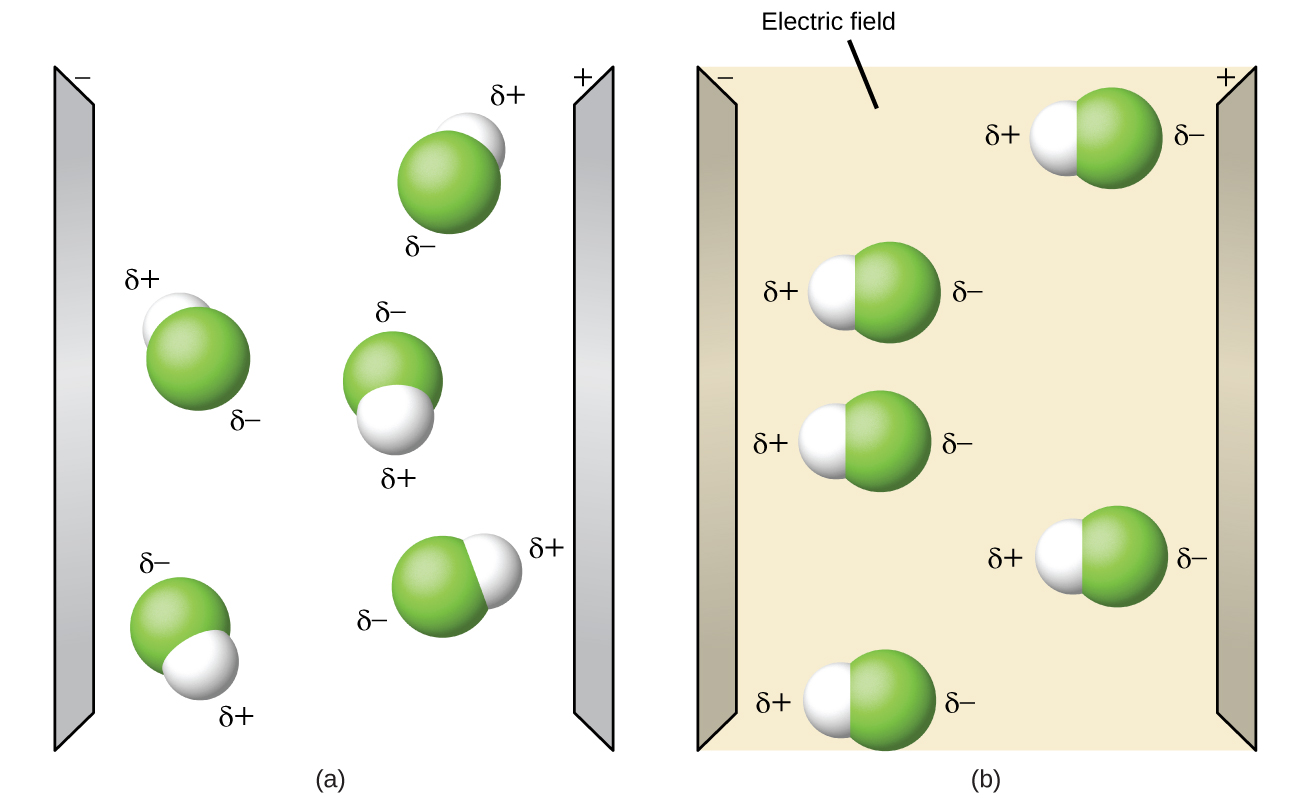
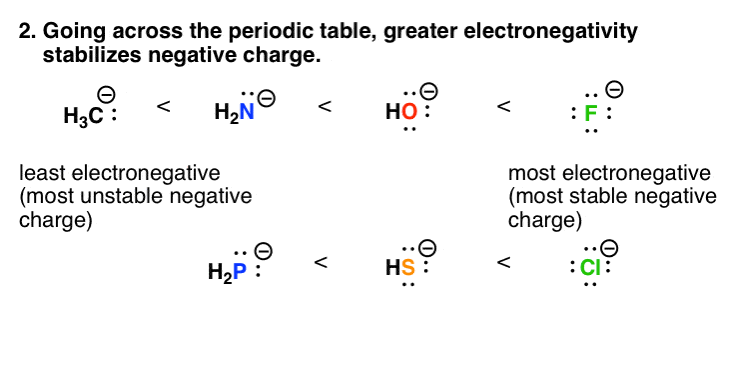
Draw a water molecule and include partial negative and positive charges. The d means that the c atom has a partial positive charge. C h en c 2 55 h 2 20 en 0 35 therefore c h is a non polar covalent bond but what about the partial negative. Figure 2 shows the distribution of electrons in the h cl bond.
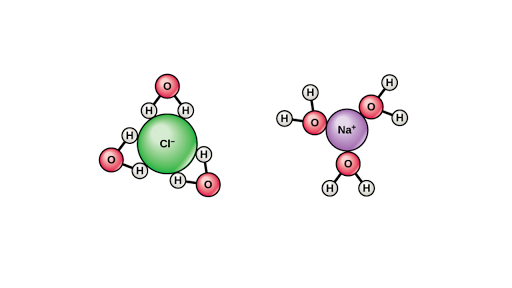
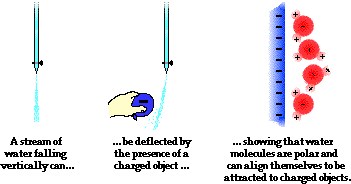
Add an electron for each negative charge remove an electron for each positive charge draw all single covalent bonds and lone pairs. Water acts as a polar solvent because it can be attracted to either the positive or negative electrical charge on a solute. In the c h bond the c is more electronegative than the h so this time the c has the partial negative charge.
Write d c h d. Water is a molecular compound. Note that the shaded area around cl is much larger than it is around h.
3 1 4 draw and label a. A water molecule is formed by covalent bonds between an oxygen atom and two hydrogen atoms. I m supposed to label the partial negative and partial positive charges on each end but i m not sure what it means.
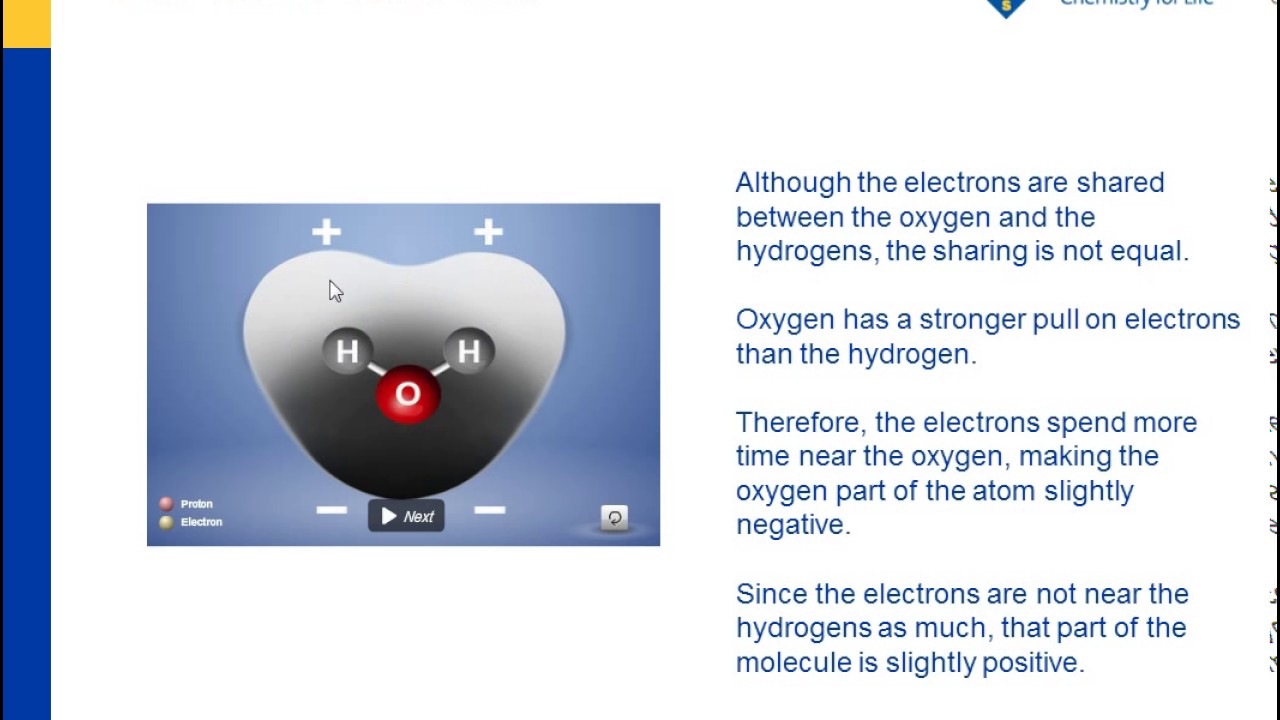
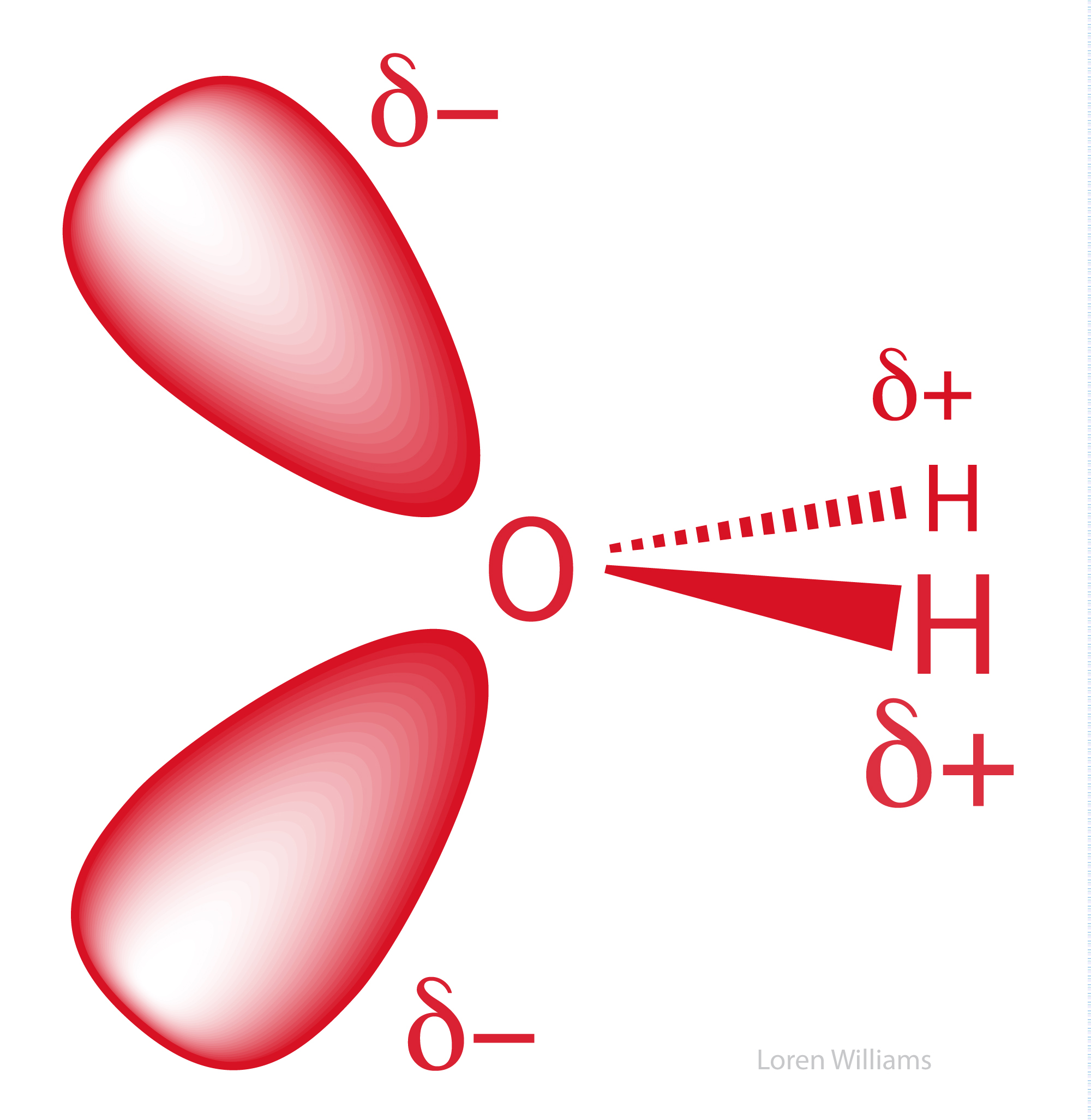
Each molecule is electrically neutral but polar with the center of positive and negative charges located in different places. Thus in an hcl molecule the chlorine atom carries a partial negative charge and the hydrogen atom has a partial positive charge. It is a polar molecule in which the oxygen end of the molecule has a partial negative charge and the hydrogen end of the molecule has a partial positive charge.
The covalent bonds are polar because oxygen is more attractive to electrons than hydrogen causing the oxygen to have a slightly negative charge and hydrogen to have a slightly positive charge. Give as many atoms as possible full octets assigning lone pairs to most. The shape of each water molecule influences the way it interacts with other water molecules and with other substances.
Compare this to figure 1 which shows the even distribution of electrons in the h 2 nonpolar bond. I ve also determined whether the bond is ionic polar covalent or non polar covalent. Has a slightly positive charge.
This will come with practice remember atom valence determine number of available valence electrons. The slight negative charge near the oxygen atom attracts nearby hydrogen atoms from water or positive charged regions of other molecules.